BJU Press’s middle and high school science product line equips students to ethically engage in the work of science through this lab-based program. Students extend knowledge and skills through strategic modeling and practice, case studies, the evaluation of scientific models, and ethics activities. Webquests, spreadsheet activities, and probeware technology build 21st century skills and relate them to the work of science. Students have opportunities to collect and analyze data and to create and evaluate their own models through guided discovery labs, inquiry labs, and collaborative STEM experiences.
Vision
To equip students to ethically engage in scientific inquiry, data analysis, and model-making so they will be able to solve real-world problems within the framework of a biblical worldview.
Goals
- Extend scientific knowledge and laboratory skills
- Guide students in applying scientific knowledge and skills in ethical ways to solve real-world problems, using activities that include collaborative STEM experiences
- Enable students to create models that describe the natural world and use them to make predictions
- Equip students with the skills to interpret informational text and apply scientific knowledge in accordance with biblical teaching
Program Approach for Science
The BJU Press middle and high school science program uses a lab-based approach to equip students to ethically engage in the work of science. Our program teaches science content from an ethical perspective based on a biblical worldview and explores what science can do through strategic modeling in inquiry labs and collaborative STEM experiences. We then direct students to use their critical-thinking and problem-solving skills to develop workable models that will help them find appropriate solutions. To that end, each chapter includes opportunities for extended study that will challenge students to harness and develop their scientific understanding and laboratory skills to serve God and to serve others. They will complete case studies, evaluate existing scientific models, and follow webquests that will require them to collect and analyze data. Ultimately, we want to equip teachers so that they can prepare a generation of student scientists who can use 21st century skills to solve real-world problems within the framework of a biblical worldview and who live in a way that’s biblically faithful.
Extending Scientific Knowledge & Skills
Students will be able to develop solutions to real-world problems only with a thorough understanding and knowledge of the sciences, scientific principles, and laboratory skills. Most elementary students will have had introductions to the sciences and valuable laboratory skills, but future science study will be more enjoyable and comprehensible if students continue to expand their understanding in each successive science course.
BJU Press begins each middle and high school science course with an overview of the work of science and foundational biblical themes that should shape a Christian’s understanding of the major issues in that field. Students will also regularly review the three-element foundation of Christian ethics: biblical principles, biblical outcomes, and biblical motivations. In addition to supporting the students’ worldview shaping, we also support their continued learning development. Our standards-based student textbooks use age-appropriate language to support learning and retention and include stunning visuals to illustrate concepts. Thoughtfully crafted chapter objectives and essential questions help students to look for key information as they read, and chapter reviews give easy-to-use bulleted reviews for each chapter.
To prepare students for college and potential careers, we highlight scientific careers and interdisciplinary opportunities related to the sciences they study and provide direction on how to pursue a field that interests them. Students need to see every opportunity they have for continued study. God uses the experiences they have in their middle and high school courses to lead them to His plan for their lives.
Applying Scientific Knowledge & Skills
Knowledge often means very little without experience. Lab activities provide a vital opportunity for students to get hands-on application of the skills they’re learning in class. In a broader sense, students gain the critical-thinking skills they need to ask better questions and create strong hypotheses by completing lab activities. BJU Press middle and high school science lab manuals are designed to help guide students through hands-on activities that build critical-thinking skills and refine students’ observational skills and their capacity to follow directions. Consistent lab work gives students an opportunity to develop and mature their way of thinking. Beyond critical thinking, each lab is also designed to accomplish specific science content learning objectives. Mini labs within the student edition and guided discovery labs in the lab manuals of BJU Press materials give students abundant opportunities for application and for practice with technology, including probeware technology.
By offering inquiry and STEM activities, we give students even more opportunities to learn hands on and apply the skills from the classroom. These activities give students ownership over the creative process, whether in groups or individually. Inquiry labs require students to use the scientific process and ask questions, form hypotheses, design investigations, analyze data, draw conclusions, communicate results, and often, ask additional questions. These activities foster curiosity and require students to think more critically than they would with traditional activities that spell out procedures and goals for them.
In addition, STEM activities require students to apply the engineering design process and use scientific inquiry, mathematical reasoning, and technology. To be successful in these activities, students must also develop 21st century skills, including collaboration, problemsolving, and communication. They learn what works, not just in the scientific process, but also with other people. BJU Press lab manual STEM activities present opportunities for students to refine their methods so that they discover more effective solutions and learn that many problems have more than one solution.
Enabling Students to Use & Create Models
Throughout the BJU Press science program, we show students how scientists use models to explain, describe, and represent the world more accurately. Models allow scientists to test their theories and apply predictions, especially when they’re working with forces and structures that are too large or too small to be observed or that no longer exist today. For example, the double-helix model of DNA brought biology to the field of molecular genetics. The heliocentric model of the solar system more accurately answered the questions proposed by observations of the night sky. We explain how historical documents, eggs, and bones help us create models and study the behavior and habitats of extinct species such as the elephant bird or dinosaurs. When scientists proposed these models, they didn’t have the resources or capabilities to prove their theories. They created their models to accurately describe the natural world and then made predictions based on their models. As we know, scientists have had to adjust existing models to accommodate new information and observations, like the heliocentric model. We teach students about models to show that science is not a progression toward greater truth. It’s a quest for more workable models.
To equip students to create and use predictive models of the natural world, student lab manuals in BJU Press’s middle and high school science program include technology-based modeling tools. Students will use graphing technology to create mathematical models and scatterplots in spreadsheet activities. Other activities use internet modeling tools to create models of molecules and atoms. Teachers can also choose activities that require students to use apps on their devices to create animated models and explore the spread and severity of viruses. They will then use the models they create to link presentations and phenomena.
Equipping Students to Interpret Informational Text & Apply Knowledge
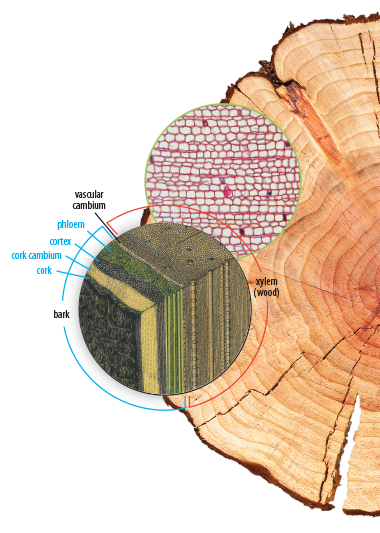
One of the biggest challenges for science students can be interacting with rich informational texts. Science-related informational texts use discipline-specific vocabulary and technical scientific diagrams. A prepared student can interpret scientific studies, engage with the information, evaluate it from a biblical worldview, and answer ethical questions presented in a study. To properly prepare and equip students, we fill our textbooks with opportunities to engage with informational texts on a high level. Not only are the textbooks themselves informational texts, but the additional recommended ethics boxes and webquests will challenge students to find more informational texts to read and learn from. Our textbooks introduce students to the vocabulary they will need, and present scientific diagrams that give students an opportunity to practice visual-analysis skills. In assigned ethics boxes and webquests, students get to apply and further develop their informational-text reading skills to real-world situations, and then they will write responses to what they have learned.
Materials
Student Edition
Each student edition introduces students to a scientific field with a solid biblical worldview foundation and a focus on real-world applications. Students will explore discipline-specific terminology and existing models for each field as well as ethical issues presented. Extensive full-color and scientifically accurate illustrations, charts, and diagrams will help students to develop a visual understanding of the concepts they study. Case studies, worldview sleuthing activities, mini-labs, ethics boxes, and questions help students think like scientists and view each scientific field from a biblical perspective.
Teacher Edition
The teacher edition for each grade offers research-based strategies, teaching notes, and suggested activities to give teachers options for daily lessons. The strategies focus on explaining concepts to students by moving from concrete to abstract and by linking scientific concepts and processes with prior learning. Each teacher edition features a suggested teaching schedule, full-color reduced student pages, icon-coded items like weblinks and demonstrations, complete answers to review questions, background information to enhance classroom instruction, and a full-year lesson plan overview. Teachers will also find active learning opportunities, inquiry activities, group discussions, formative assessments, and intriguing chapter openers to add depth and variety to their daily teaching plan.
Student Lab Manual/Activities
Each lab manual or student activities manual gives students opportunities to develop laboratory skills and apply what they have learned in real-world situations. Students will solidify their understanding of concepts by connecting the content with real-world problems. Discovery labs give guidance for exploring God’s world; inquiry labs require students to use the scientific process to create their own activity; and STEM activities develop key science, engineering, and problem-solving skills through observing, recording, and analyzing samples and data to make models. Students then test those models to understand their workability.
Teacher Lab Manual / Activities Answer Key
The teacher lab manual and answer key contains full-color, reduced-size lab manual pages with answers as well as additional instructions on preparation and the safe execution of lab exercises.
Assessments
The assessment packets provide summative assessment opportunities to measure students’ knowledge and understanding of key concepts. The tests and quizzes include opportunities for students to infer information from images, and they assess students’ recall and higher-order thinking skills. An assessments answer key is available for each grade.
Scope & Sequence
General Science
Process skills, science tools, scientific method
Earth Science
Earth
Earthquakes: faults, causes, recording and interpreting data from earthquakes; Volcanoes: causes, locations, classifying by shape and eruption, effects and products of volcanoes; Weathering and erosion: types and examples of mechanical and chemical weathering, agents of erosion, types of erosion; Soil: particles and texture of soil, formation, horizons; Natural resources: renewable and nonrenewable energy resources (fossil fuels, nuclear energy, hydroelectric energy, geothermal energy, wind energy, solar energy), minerals, metals, soil conservation, water, reduce, reuse, recycle
Space
Stars: magnitude, size, distances between, kinds of stars, constellations, star groups, asteroids, meteoroids, comets, telescopes, spectroscopes; Solar system: parts of the sun, solar storms, seasons, the planets, dwarf planets, eclipses, space exploration, satellites, probes
Life Science
Living Things
Cells and classification: characteristics of living things, cell theory, tissues, organs, systems, cell reproduction, six kingdoms of classification, scientific names, microscopes; Animal classification: invertebrate phyla (Porifera, Cnidaria, echinoderms, mollusks, worms, arthropods), vertebrates (fish, amphibians, reptiles, birds, mammals); Plant classification: nonvascular plants (mosses and liverworts), seedless vascular plants (ferns, horsetails, and club mosses), gymnosperms, angiosperms, parts of a plant; Plant and animal reproduction: parts of a lower, pollination and fertilization, types of fruit, seeds, spores, asexual reproduction, gestation, placental and marsupial mammals, eggs, parental care; Genetics: heredity, traits, DNA structure, Mendel's experiments, dominant and recessive genes, Punnett squares, genetic disorders and diseases, genetic engineering
Human Body
Nervous system: central nervous system, the brain, the peripheral nervous system, neurons, reflexes, the five senses, memory, sleep, disorders, drug abuse, endocrine system; Immune system: communicable and noncommunicable diseases, pathogens, vectors, epidemics, nonspecific responses, the immune response, functions of white blood cells, immunity, antibiotics, antibodies, autoimmune diseases, allergies, transfusions and transplants, immune deficiencies
Physical Science
Motion
Motion and machines: velocity, acceleration, momentum, Newton's laws of motion, work, simple machines (levers, pulleys, wheel and axle, inclined planes, wedges, screws), compound machines
Energy
Electricity: static and current electricity, types of circuits, measuring electricity, batteries, magnetism, electronics, integrated circuits, computers
Matter
Chemistry: parts of an atom, atomic theory, classifying elements, periodic table of the elements, compounds, chemical formulas, chemical reactions, atomic bonds, acids and bases
The Pattern of Life
Definition of science, science and worldview, biblical vs. naturalistic worldviews, science and biblical ethics, characteristics of life, homeostasis, design of life, modeling, thinking scientifically, limitations of science, classification of life; cell theory, cell structure and function, cellular respiration, photosynthesis; genes, DNA replication, RNA transcription, protein synthesis, cell division, mitosis and meiosis; Mendelian genetics, genetic crosses, variations on simple genetics, population genetics; biblical creationism vs. evolutionism, change in nature, worldview and change
Microorganisms and Plants
Archaebacterial vs. eubacteria, bacterial structure, reproduction, and importance; antibiotic resistance in bacteria; viruses; protist movement, nutrition, classification, and reproduction; structure, nutrition, and importance of fungi; plant structure and classification; plant hormones, tropisms, and photoperiodism, plant reproduction and life cycles
The Animal Kingdom
Characteristics of animals, characteristics and classifications of invertebrates; sponges, cnidarians, worms, mollusks, echinoderms, arthropods; characteristics and classifications of vertebrates; endotherms vs. ectotherms; fish, amphibians, reptiles, birds, and mammals; nutrition, transport, support, movement, and control systems of animals; animals reproduction and behavior, external and internal fertilization, egg structure and development, and placental reproduction; innate and learned behavior
The Human Body
Structure and function of skin, bones, joints, and muscles; types of muscles; digestive system structure and function; food and nutrition; chemical vs. mechanical digestion; alimentary canal organs; accessory organs; urinary system structure and function; respiratory system structure and function; connection between the respiratory and circulatory systems; circulatory system structure and function; heart, blood cells and plasma; blood vessels; flow of blood through the heart and lungs; connection between the circulatory and lymphatic systems; lymphatic system and immunity; lymph vessels and nodes; components of the immune system; nonspecific vs. specific immunity; vaccines; actives vs. passive immunity; parts of the nervous system; central nervous system vs. peripheral nervous system; nerves, reflex arc, and nerve impulses; sense organs structure and function; eyes, ears, touch, smell, and taste; hormones and endocrine glands; puberty; human reproduction and biblical sexuality; human growth and development
Interacting with the Biosphere
Ecology; abiotic vs. biotic factors; ecosystems and biomes; cycles of matter; water cycle, oxygen and carbon cycles, and nitrogen cycle; food chains, energy pyramids, and food webs; relationships between organisms; symbiosis; succession; managing and protecting the environment; pollution classification and solutions; substance vs. energy pollution; using natural resources; renewable vs. nonrenewable resources; management philosophy; conservation vs. preservation; management principles
Introduction to Earth Science
Earth science and exercising biblical dominion, worldviews and science, the structure of science, scientific models, what earth science is; maps and cartography, geographic information systems (GIS); introduction to physical science, matter, forces, energy, and measuring
The Restless Earth
Earth as a special place designed for life, a brief history of geology, operational and historical geology, the earth’s interior structure, natural resources; old- and young-earth origin theories of the earth, evidences for catastrophic changes in earth’s history, models for geologic tectonics; tectonic forces, faults and earthquakes, earthquakes and seismology, effects of earthquakes; mountains and hills, tectonic mountains and landforms, nontectonic mountains and landforms; volcanic emissions, volcano activity and classification, intrusive volcanism
Earth’s Rocky Materials
Describing minerals, identifying and classifying minerals, minerals as resources; classifying rocks, igneous rocks, sedimentary rocks, metamorphic rocks, critiquing the uniformitarian rock cycle; the process of fossilization, paleontology, fossil fuels; weathering, erosion and deposition, soils and soil formation
The Water World
Ocean basins and landforms, seawater composition, ocean environments; tides, currents, waves; history of oceanography, methods and instruments, deep-sea exploration, underwater habitats, research vehicles; stream characteristics, lakes and ponds, limnology; groundwater reservoirs, groundwater chemistry, water as a resource, solution caves and karst topography
The Atmosphere
Composition and thermal structure of the atmosphere, special regions; energy in the atmosphere; measurable weather data, causes of wind, global wind patterns, sources of local winds, cloud formation, classifying clouds, precipitation, dew and frost; air masses and weather fronts, causes of precipitation, winter storms, thunderstorms, tornadoes, hurricanes, weather forecasting, weather maps, applications of GIS in weather modeling; describing climate and climate zones, climate data and interpretation, observed short-term climate changes from volcanism and oceanic cycles, climate models, worldviews and long-term climate change, environmentalism and biblical stewardship of the environment
The Heavens
The sun-earth-moon system—the sun’s structure, composition and energy, the solar spectrum; the moon’s structure and surface, origin theories; Earth’s orbit, seasons, timekeeping, lunar phases, eclipses, tidal effects; models of the solar system, Kepler’s laws, classification and brief description of the planets, dwarf planets, small solar system bodies, evidences for a young solar system, constellations and star properties, stellar classification and the H-R diagram, stellar aging, classification of galaxies, nonstellar objects, cosmology and worldviews; challenges of space exploration, rocketry, principles of satellite and space probes, challenges and need for manned space exploration
Structure of Matter
Presents science as the development of models to explain and describe phenomena in a fallen and broken world; biblical versus secular worldview aspects of science; definition of key elements of scientific knowledge—laws, theories, and hypotheses; scientific study and application of scientific knowledge as a key aspect of obedience to the Creation Mandate; methodologies of science; scientific measurement; the metric system; accuracy, precision, and repeatability in measurements; introduction to the nature and classification of matter and energy; changes matter undergoes; historical development of the atomic model; structure of the atom; origin of the periodic table; elements and their symbols; classification of the elements; periodic trends; electronegativity and valence electron structure; covalent, ionic, and metallic bonds; compounds classified according to bond-type; chemical formulas and equations; oxidation numbers; introduction to organic chemistry and biochemistry
Changes in Matter
Types of chemical reactions; radiation and nuclear changes; classifying mixtures; solutions and the solution process; measuring concentration; acids and bases; salts from acid-base reactions; pH system and measurement
Matter in Motion
Describing motion; frames of reference; momentum; Newton’s laws of motion; gravity; free-fall; mechanical work; levers and other simple machines; mechanical advantage and efficiency; kinetic and potential energy; energy transformations and conservation; thermodynamics— thermal energy, temperature, and heat; basic hydraulic theory; gas laws; fluid mechanics
Waves and Energy
Description of periodic motion; waves and wave phenomena; sound and its properties; the human voice and hearing; applications of sound; static electricity; electric fields; electric current and Ohm’s law; circuits and electrical safety; magnets and magnetism; AC and DC generators and motors; transformers; electromagnets and their uses; bands of the electromagnetic spectrum; the properties of visible light; the nature of color; reflection and mirrors; refraction and lenses
Science of Life
Creation, Fall, Redemption, the Creation Mandate, study of life, attributes of life, the energy and information of life, worldviews, nature of science, modeling, scientific method, elements, thermodynamics, basic chemistry, physical and chemical changes, solutions, organic chemistry, biochemistry, ecology, ecosystems, biomes, food web, symbiosis, biochemical cycles, population growth and biodiversity, climate change, conservation, cell theory and structure, organelles, homeostasis, osmosis, membrane transport, metabolism, DNA synthesis, protein synthesis, photosynthesis, aerobic cellular respiration, fermentation, mitosis, meiosis, Mendelian genetics, genetic crosses, sex-linked traits, gene expression, population genetics, gene and chromosomal mutations, cancer, genetic engineering, historical development of biological evolution, tenets of biological evolution, biblical views of origins, contrasting the evolutionary and biblical views of history
Science of Organisms
Taxonomy, binomial nomenclature, use of dichotomous keys, comparison of species and kind, speciation, phylogenetic trees, archaearchaea and bacteria, bacterial structure, bacterial reproduction, control of bacteria, viruses and related organisms, diseases, protozoan classification and structure, protozoan reproduction and role in the environment, chromist classification and structure, chromist reproduction and role in the environment, evolution and protists, fungi classification and structure, fungi reproduction and role in the environment, plant classification and structure, nutrient transport in plants, plant hormones, tropisms, plant reproduction and role in the environment for mankind’s use, characteristics of animals, classification and structure of sponges, cnidarians, worms, mollusks, echinoderms and arthropods, invertebrate reproduction and role in the environment, classification and structure of ectothermic vertebrates, the reproduction and role in the environment for fish, amphibians, and reptiles, classification and structure of birds and mammals, the reproduction and role in the environment of endothermic vertebrates
Study of Human Life
The essence of humanity; tissues, organs, and systems; structure, function, and role of the following systems in the human body: integumentary, lymphatic, skeletal, muscular, respiratory, circulatory, digestive, excretory, nervous, endocrine, and reproductive systems; human growth and development, balanced living
Foundations of Chemistry
Chemistry: modeling matter, chemistry and worldview, chemistry and modeling, chemistry helps people, a biblical worldview of chemistry, doing chemistry, scientific inquiry, thinking like a scientist
Matter
Classification of matter, organizing our study, properties and changes of matter, classification of matter, energy and matter, work and energy, conservation of mass-energy, the law of entropy, thermal energy, temperature, and heat, states of matter, changes of state
Measurements in Chemistry
Measurement systems, metric system, unit conversion, measurements, limitations of measurements, accuracy, precision, significant figures, problem solving in chemistry, calculations with measured data
Atomic Structure
Early thoughts about matter, investigating atoms, Dalton’s model, development of atomic models, Thomson’s model, Rutherford’s model, completing Rutherford’s model, useful notations, isotopes
Electron Arrangement
Bohr model, electron energy levels, the quantum mechanical model, electron configurations, valence electrons, electron dot notation, ions
Periodic Table and Elements
Early organization, element periodicity, Mendeleev’s periodic table, the modern periodic table, periodic trends, elements by their groups
Chemical Bonds
Bonding basics, octet rule, types of chemical bonds, polarity and bond character, covalent bonding, diatomic elements, Lewis structures, ionic bonding, the structure of ionic compounds, polyatomic ions, metallic bonding, properties of compounds, using chemistry to solve problems
Bond Theories and Molecular Geometry
Bond theories, limits of Lewis structures, orbitals and valence bond theory, molecular resonance, when the octet rule doesn’t work, molecular orbital theory, molecular geometry, VSEPR and molecular shape, orbital hybridization, a measure of polarity, water molecules designed for usefulness, seeking the perfect bonding model
Chemical Compounds
Ionic compounds, oxidation numbers, using oxidation numbers, polyatomic ions, covalent compounds, nonmetals with multiple oxidation numbers, writing chemical formulas, naming compounds, acids, binary acids, ternary acids
Chemical Reactions and Equations
Chemical equations, information in chemical equations, balancing equations, special symbols in equations, limitations of balanced equations, types of reactions, ionic equations
Chemical Calculations
The mole, Avogadro’s number, molar mass, types of formulas, percent composition, empirical formulas, stoichiometry, limiting reactants, percent yield
Gases
Properties of gases, kineticmolecular description of gases,properties of gases, gas laws, standard conditions, Dalton’s law of partial pressures: mixtures of gases, gas stoichiometry, gases in reactions, molar volume, ideal gases, ideal gas law
Solids and Liquids
Intermolecular forces, kinetic description of solids, crystalline and amorphous solids, crystalline structures, kinetic description of liquids, effects of intermolecular attractions, vapor pressure and boiling point, distilling liquids, phase diagrams, using liquids to solve problems
Solutions
The dissolving process, types of solutions, the dissolving process, solvent selectivity, solution equilibria, rate of solution, solubility, measures of concentration, colligative properties, suspensions and colloids, properties of colloids
Thermochemistry
Thermodynamics and physical changes, measuring heat and temperature, enthalpy of phase changes, specific heat, thermodynamics and chemical changes, reaction tendency, chemical bonds and enthalpy, entropy and reaction tendency, entropy changes, free-energy change, worldview conflict in thermodynamics
Chemical Kinetics
Reaction rates, kinetics, energy diagrams, collision theory, activation energy and the activated complex, rates of reactions, reaction mechanisms, rate laws and reaction orders, kinetics in the real world
Chemical Equilibrium
Equilibrium, equilibrium constants, le Châtelier’s principle, equilibria and industry, solution equilibrium, ionic equilibria, common-ion effect, precipitation reactions
Acids, Bases, and Salts
Defining acids and bases, properties of acids and bases, models of acids and bases, acid-base equilibria, self-ionization of water, pH and pOH scales, acid-base strength, amphoteric substances, polyprotic acids, measuring pH, neutralization, salts, titration, buffers
Oxidation and Reduction
Redox reactions, oxidation, reduction, oxidizing and reducing agents, using oxidation to solve problems, balancing redox reactions, electrochemical reactions, electrochemical cells, electrolytic cells, voltaic cells
Organic Chemistry
Organic compounds, unique carbon atom, classification of hydrocarbons, substituted hydrocarbons, alcohols, ethers, aldehydes and ketones, carboxylic acids, esters, amines and amides, organic reactions
Biochemistry
Chemistry of life, chemical reactions in cells, biochemistry and ultimate questions, carbohydrates, lipids, proteins, polypeptide chains, enzymes, nucleic acids, amino acids, worldview conflict in biochemistry
Nuclear Chemistry
Inside the nucleus, nuclear stability, energy and nuclear changes, measuring radiation, radioactive decay, predicting types of decay, radioactive decay series, half-life, using nuclear chemistry, nuclear reactions, fission, fusion, using nuclear chemistry to solve problems
Kinematics
The biblical worldview in which we do science in obedience to God’s commandment to exercise good stewardship over the earth for His glory and for the benefit of our fellow humans; the structure and limitations of science; overview of physics; scientific methodology and modeling; the metric (SI) system of measurement as well as principles of measurement; rules for determining and using significant figures in measurements and calculations; mathematical description of motion in one and two dimensions; vectors and scalars in graphical and analytical solutions
Dynamics
Force and the causes of motion according to Newton’s laws (dynamics); friction; motion in a plane, including circular motion; motion of multibody systems; work, energy, and total mechanical energy; conservation of energy; momentum and its conservation, collisions, center of mass, and angular momentum; periodic and simple harmonic motion, the pendulum, damped and driven oscillations, physical waves, and sound
Thermodynamics and Matter
Thermal properties of matter, measuring temperature, and the gas laws; theories of heat, thermal energy, mechanisms for heat transfer; the four laws of thermodynamics; entropy and its consequences; fluid mechanics (hydrostatics and hydrodynamics)
Electromagnetics
Electrostatics and charges; electric fields and capacitors; current, voltage, resistance, and basic DC circuits; magnetism and its relationship to current and conductors; and electromagnetism and alternating currents
Geometric Optics and Light
The electromagnetic spectrum, sources and propagation of light; intensity and color of light; reflection and mirrors; refraction and lenses; and wave interference, diffraction, and polarization
Modern Physics
Relativity: Galilean, special, and general relativity; quantum physics: quantum theory, quantum mechanics, the atom, and modern atomic models; nuclear physics: radiation and radioactivity, radioactive decay, nuclear reactions, and subatomic particles
Biblical Worldview Shaping
- Identity—Relating personality and self-image to overall health
- Relationship—Applying biblical principles to family and peer relationships to promote personal and community health
- Discernment—Evaluating the reliability of health information, products, and services
- Ethics—Formulating positions on health-related issues, such as reproductive issues, organ donation, and disabilities
- Virtue—Applying biblical principles to become healthy and holy people
Life Science
- Defining health—Health care basics; Health risks; Health skills; Health literacy
- Health and safety—Personal hygiene; Assessing risks; Personal safety; Basic first aid
- Mental health—Personality; Self-image; Responding to stress; Mental disorders; Medical model and biblical model of treatment; Mental health’s effect on wellbeing; Suicide
- Health and life management—Money management; Budgeting; Decision-making; Character; Motivation and success; Communication skills; Empathy; Resilience; Coping skills; Handling loss
- Social health—Family relationships; Peer relationships; Making positive choices; Helping others; Preventing violence; Resources for abuse victims; Self-defense; Media and technology; Technology and health
Life Science: The Human Body
- Structure, function, and role of the human systems—Immune system; Skeletal system; Muscular system; Pulmonary system; Cardiovascular system; Digestive system; Urinary system; Nervous system; Endocrine system; Reproductive system
- Organ donation
- Diseases—Infectious diseases: origin, immunity, and disease prevention; Chronic diseases: cardiovascular disease, risk factors, cancer, risk reduction, respiratory diseases, diabetes, and autoimmune diseases
- Disabilities—Impairment; Accessibility and accommodation; Cognitive disabilities; Intellectual disabilities; Learning disabilities; Assistive technology; Prenatal discrimination; Hearing disabilities; Visual disabilities; Physical disabilities; Inclusion
- Drug use and abuse—Medication: uses, classification, prescriptions, medication adherence, drug reactions, side effects, polypharmacy, interactions, safety, abuse, addiction, reducing risks, and misuse; Substance abuse: alcohol, risktaking, avoidance, effects of marketing, impact, nicotine, vaping, benefits of stopping, impacts, illegal drugs, classifying, risk-taking, avoidance, impacts, and resisting peer pressure
- Growth and development—Nutrition: Macronutrients and micronutrients, Carbohydrates, proteins, and lipids, Hydration, Vitamins and minerals, Health nutrition choices, Nutrition labels; Fitness and exercise; Reproductive health: Gender, Menstrual cycle, Birth control, Abstinence; Human development: Pregnancy and infertility, birth, prenatal care, risks to the unborn, and abortion, Infancy and child development, Adolescence, Early adulthood, Marriage and parenting, Stages of adulthood
Earth and Space Science
- Environmental health—Air quality; Noise pollution; Light pollution; Land pollution; Water pollution; Clean water; Fracking; Types of natural disasters, disaster preparedness; Stewardship; Conservation